Flipping the “Switch”: Big Pharma’s Biggest Challenge
Drug companies believe they’ll reap billions taking prescription medicines “over the counter.” But the strategy is no substitute for real innovation.
(originally published by Booz & Company)
 |
|
Illustration by Lars Leetaru |
The industry definitely has the itch to switch. Virtually all consumer health-care companies assume switches will contribute significantly to over-the-counter (OTC) revenue growth in the near future — possibly creating new categories while reinvigorating such old warhorses as cold, cough, and allergy treatments. But our own extensive study of consumer health-care research and development shows prospects are less sanguine. Of the $50 billion worth of prescription sales derived from drugs that are losing patent protection over the next five years, we conclude that, at best, only about $3 billion can be captured by switches. This translates into a 2 percent compound annual growth rate over the next decade — in an industry that needs far higher growth to satisfy shareholders.
Our research provides compelling evidence that OTC industry players will have to perform a massive overhaul of their innovation engines to compete in the coming years. Our study, which consisted of a detailed questionnaire followed by a series of interviews with company managers (ultimately, nine of the 11 major consumer health-care companies elected to participate in exchange for a copy of the 200-page report and a follow-up briefing), also holds implications for a range of consumer products companies, many of which face economic and market forces that are compelling them to kick-start creativity.
A Fragmented Industry
Although OTC represents only about 3 percent of total U.S. health-care spending, it’s still big business, reaping almost $30 billion in sales last year. Its major categories are cold and cough (24 percent of the market), analgesics (22 percent), and digestive remedies (20 percent), followed by medicated skin-care products, smoking cessation aids, and eyedrops. The industry remains fragmented, with Wyeth Consumer Healthcare (formerly Whitehall-Robins Healthcare/American Home Products) and McNeil (owned by Johnson & Johnson) each owning approximately 10 percent of the market, and runners-up Pfizer and GlaxoSmithKline holding just more than 8 percent each. Despite fragmentation, individual brands do command attention in certain segments. McNeil’s Tylenol has more than 17 percent of the analgesics market, for instance, and Wyeth’s Robitussin commands about 10 percent of the cold and cough category.
Not surprisingly, most of the major companies in the industry are owned by much larger pharmaceutical “druggernauts”; of the top 11 consumer health-care players, only two (Procter & Gamble and Johnson & Johnson’s McNeil) are part of companies known for consumer products. Ownership by big pharma has critical repercussions for the entire industry, the most significant being that OTC divisions — which generate 10 percent of parent companies’ sales at most — often feel like unloved stepchildren. (As one consumer health-care manager told us during our study, “We’re a pimple on the back of big pharma.”) Over the last five years, while the pharmaceutical industry’s top line has averaged 13.5 percent annual growth, consumer health care has limped along at 3 to 4 percent, or about the pace of inflation plus population growth.
In many ways, consumer health-care companies are at the mercy of forces beyond their control. Strict FDA regulations make product differentiation on the basis of active ingredients all but impossible; some 100,000 OTC products are on sale in the U.S., but they contain fewer than 1,000 distinct compounds. Every major cough syrup, for instance, contains some variation of the same five active ingredients. The consequence is that private-label brands like Walgreens’s Wal-Tussin don’t just look and taste like Robitussin — they are Robitussin. Unable to create truly new products, OTC players are trapped in a cycle of line extensions, new claims, and line extensions of new claims, as, for example, Excedrin becomes Extra-Strength Excedrin becomes Excedrin Migraine, and so on. In this benighted world, when Pfizer’s Benadryl managed to eke out 0.3 percent share growth in 2000 by launching Benadryl Fastmelt tablets for kids, it was considered a major success story.
OTC Myths and Truths
Pharmaceutical companies proffer several reasons for owning such low-growth subsidiaries. Consumer health-care firms, they argue, have mass marketing skills, which pharmaceutical companies have historically lacked; they provide access to branding opportunities, shelf slots, and cross-marketing; they are an inlet into a less volatile sector with close ties to large retailers. Moreover, consumer subsidiaries can offer greater control over the entire life cycle of the drug, including its post-patent maturity. But are these arguments convincing?
First, “branding” in the OTC space has been oversold. It would be difficult to find a more highly valued brand than Tylenol, for instance, or a more respected marketing machine than Johnson & Johnson’s. Yet Tylenol is currently losing market share at a rate of 1 percent annually. The reason: Year after year, more and more consumers learn to harness the curative powers of the higher-priced “names” from lower-priced, identical private-label versions.
Second, channel access isn’t what it used to be. As discount retailers such as Wal-Mart steal share from pharmacy chains and grocery stores, OTC consumers are increasingly shopping in outlets that prefer to stock and sell private-label drugs, which have much higher margins and even benefit from free piggyback advertising (because of copycat packaging and homophonous names). The result is that the discounters’ share of the cold and cough category, for example, grew 5 percent last year, while drug and grocery outlets each lost 3 percent.
The third reason pharmaceutical companies own consumer health-care subsidiaries — albeit a rationale they’re reluctant to hype very publicly — is their belief in the potential of the OTC switch. “The only way [our parent company] can justify owning us,” one interviewee told us, “is because we make it easier to switch.”
In a perfect world, the switch can sing a loud song of symbiosis and synergy, orchestrating big pharma’s R&D brawn with OTC’s marketing might into a gorgeous opera of growth. Consider the anecdote of Advil. As patent expiration for prescription ibuprofen (branded Motrin) neared in 1984, Upjohn partnered with Bristol-Myers Squibb to sell an OTC version under the brand name Nuprin, while Britain’s Boots licensed American Home Products to sell its own version, Advil. AHP benefited from a marked advantage — its ability to explicitly mention the Motrin brand name in its advertising, a privilege contractually denied Bristol-Myers Squibb. Within 10 years of its launch, Advil held 17 percent of the OTC analgesics market, decimating both OTC Nuprin, which eked out a bare 3 percent share, and prescription Motrin.
But the switch is a strategy of fits and starts — an unstable ground on which to build a business. In the real world, outside big pharma’s star chambers, little recent evidence shows that switching will pay. The rate of switches has been declining since the mid-1990s. After peaking at eight in 1996, the number approved by the FDA fell to six in 1997 and three each in 1998 and 1999. There was only one switch in 2000 and 2001 combined, and as of this writing, there have been none in 2002. (See Exhibit 1.)
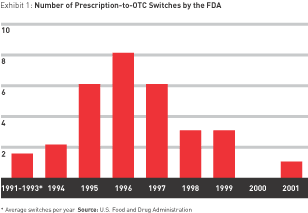
Although there is some indication that the drought may be ending — Schering-Plough has announced its intention to sell Claritin over the counter as early as fall 2002, inspiring other prescription allergy medications (Allegra and Zyrtec, for example) to follow suit — the future of switching does not appear to be much more promising than its recent past.
Because the pool of potential switches is known, marketers can build realistic scenarios. Starting with the universe of all switch candidates (which we’ll limit to drugs currently nearing the end of their patent life cycle, because virtually all switches have taken place on or about the date of patent expiration), it’s possible to forecast the approval process long established by the Food and Drug Administration (FDA). The common-sense criteria for “switchability,” according to the FDA, are: The drug must treat a condition that is not incredibly serious (e.g., a headache), and is fairly easy to self-diagnose and self-treat. In addition, the treatment must be easily self-administered (e.g., a pill).
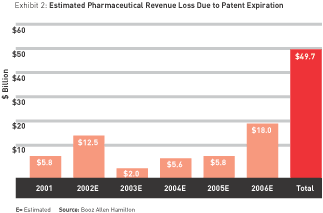
Since patent expiration dates are readily available, it is possible to determine how much revenue will be lost as a result of patent expiration in the near future. (Patent protection in the United States lasts 17 years, although it can be extended through various legal maneuvers.) We estimate drug companies will lose a total of about $50 billion in revenue due to prescription-product patent expirations between 2001 and 2006. (See Exhibit 2.) As we look ahead to 2010, estimated revenue loss for the top 14 pharma companies increases to a total of $85 billion. Those most affected are the industry’s current sales leader, Pfizer ($15 billion), GlaxoSmithKline ($14 billion), and Merck and AstraZeneca (about $11 billion each).
As we consider the $50 billion in revenues from prescriptions whose patents will be expiring over the next half-decade, the FDA “filters” can help determine how many prescription products are likely to actually make the transition to OTC. The likeliest candidates are drugs in categories that have already been switched, or that the FDA has publicly stated have a readiness to switch; these are categories in which safety and efficacy are firmly established and consumer ability to self-diagnose and self-treat is unassailable (examples include allergy, migraine, and yeast-infection treatments). On the other hand, certain drugs will never switch because the conditions they treat are simply too serious and require a doctor’s supervision (cancer, Parkinson’s, and Alzheimer’s, for example).
The mystery zone lies between these two poles — drugs in categories that have not yet been switched and have no history of consumer safety to buttress their candidacy, yet that treat disease states that are comparatively benign and manageable, at least in the short run. Many OTC companies are pinning their hopes on opening up such new categories, which include medications for hypercholesterolemia (i.e., high cholesterol), hypertension, asthma, arthritis, and benign prostatic hypertrophy (BPH). Statins treating high cholesterol are an area of particular interest, with two likely switch candidates: Merck’s Mevacor and Bristol-Myers Squibb’s Pravachol. Although the FDA issued an unambiguous “guidance” in 1997 stating that such drugs should be available only by prescription, the agency has subsequently given indications that its hard line may be softening.
It is likely that drugs with a long history of safety and efficacy in their prescription-only form, such as statins, could indeed switch despite FDA concerns. Categories treating relatively mild chronic conditions (e.g., arthritis, obesity) have a better chance of going OTC than those treating more serious, acute conditions (e.g., irritable bowel syndrome). And activists with a pressing public health case, such as the need for asthma medication to treat an epidemic among inner-city children, may find ways to convince the FDA to lower its usual thresholds. In constructing a best-case scenario, we decided to give the OTC industry the benefit of the doubt, and assumed the FDA would approve switches for drugs in the following “borderline” categories: high cholesterol, hypertension, osteoporosis, BPH, and a handful of very common, mild viruses (e.g., rhinovirus). Remember this is a best-case scenario; our assumptions are very aggressive given the current regulatory climate.
Adding up the total prescription sales in 2001 of drugs whose patents expire in the next five years — and that belong to one of the more easily switchable categories cited — we arrive at a total of $15 billion in what we might call “potentially available revenue.” That is, about $15 billion worth of current pharmaceutical revenue could conceivably be switched in the next half-decade.
To an industry scaring up about $30 billion yearly, $15 billion is a nice piece of change. Unfortunately, there is a problem: $1 in prescription pharmaceutical revenue does not equal $1 in OTC revenue. In the U.S., prescription drugs are generally reimbursed, leading to very low price sensitivity among the insured population; consequently, pharmaceutical companies are more than able to offset high development costs with significant markups. The question relevant to our analysis is: How much revenue is lost when a drug gets switched? A recent Booz Allen Hamilton analysis indicated that, historically, an OTC drug earns about 20 percent of what its prescription predecessor earned. So $15 billion in “available revenue” becomes more like $3 billion in actual OTC revenue spread over five years.
That figure, however, understates the risks and costs of actually attempting a switch. Switching a prescription drug is among the most expensive endeavors a consumer health-care company can undertake. Switch candidates can spend upward of five years in the clinical-trial pipeline, accumulating evidence that consumers can indeed diagnose and treat the condition themselves. A routine switch can end up costing $30 million to $50 million to shepherd to market. Considering that even a successful OTC company, such as the Johnson & Johnson–Merck joint venture that markets Pepcid AC, reaps only about $200 million in total revenues from switched products, development costs of this magnitude are very, very real.
We now have a convincing best-case scenario for total switch revenues over the next five or so years. We have seen how an assessment of switchable drugs whose patents are expiring yields a potential revenue pool of $15 billion. We have seen how this $15 billion drops to $3 billion because of OTC economics. All that remains is to translate this incremental revenue into a growth rate. The bottom line: Switches could yield at most an incremental 2 percent in annual top-line growth to the consumer health-care industry, with net profit growth being substantially lower. Added to recent historical growth rates, this 2 percent bumps the figure up to 5 percent — still far short of the pharmaceutical industry’s expectations.
Nimble Winners
Is the switch, then, a quixotic quest, a drug-fueled dream? Perhaps not. Although we have shown that switches will not “save” the OTC industry, a handful of nimble players with top-tier switch capabilities — the Johnson & Johnson–Merck joint venture, say, or Wyeth Consumer Healthcare — could move fast, execute precisely, and win a larger share of the wealth. In addition, our analysis rests on a number of assumptions, including a presumption of stability in pharmaceutical-industry economics, that could be proved false. If, for example, the government mandates (or insurers adopt) some form of full or partial reimbursement for nonprescription drugs, the OTC upside — and the prospects for switches — could improve dramatically.
What are the implications of this analysis for the consumer health-care industry? We believe all consumer health-care companies must aggressively ramp up their switch capabilities while pushing into innovative growth platforms. Platforms most often mentioned during the course of our study included expanding into “specialty pharmaceuticals” (i.e., niche drugs), focusing on disease state management, and inventing novel delivery mechanisms and “organoleptics” (i.e., the feel and texture of the drug itself). Best-in-class survey participants were actively investing in multiple platforms quite different from their historical line extensions and new flavors.
Although our study could not prescribe a silver bullet for the consumer health-care industry’s ills, it did make one clear diagnosis: Accelerating real growth will not be as easy as flipping the “switch.” ![]()
Reprint No. 02401
| Authors
Minoo Javanmardian, javanmardian_minoo@bah.com Minoo Javanmardian is a senior associate with Booz Allen Hamilton in Chicago. She focuses on growth and innovation strategies in the health-care industry, including pharmaceutical, insurance, and consumer health-care companies. Alex Kandybin, kandybin_alex@bah.com Alex Kandybin is a principal with Booz Allen Hamilton in New York. He concentrates on operations and innovation strategy in the consumer products and health-care industries. Martin Kihn, kihn_martin@bah.com Martin Kihn is an associate with Booz Allen Hamilton in New York. He has worked in the consumer products, retailing, and media industries. His articles have appeared in the New York Times, New York, and Forbes. |



