Smarter Medicine
How the U.S. Centers for Disease Control and Prevention revolutionized the way vaccines are delivered.
(originally published by Booz & Company)
 |
|
Photograph © Jim West/Alamy |
If you’re looking for a public health success story, it would be hard to do any better than the Vaccines for Children (VFC) program. Then President Bill Clinton and the U.S. Congress enacted the VFC legislation in 1993 with a simple goal: to provide federal funds so that all U.S. children could have access to the recommended vaccinations at no cost. Since then, the rate of overall immunization coverage has risen significantly and is now at record levels; 90 percent of 2-year-olds are immunized against the major childhood diseases. Thanks to the VFC program, which provides about 43 percent of all childhood vaccines in the country, most virulent infectious diseases have been nearly eliminated in the United States. The program is one of two national immunization programs run by the Centers for Disease Control and Prevention (CDC). The VFC’s success is a tribute to years of perseverance and dedication on the part of the countless public health professionals who have built the program into the CDC’s biggest — and into a leading example of a thriving public–private partnership.
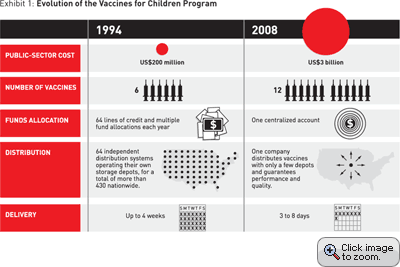
Of course, any program that extends into every state and U.S. territory bumps up against concerns. In the case of VFC, the details of implementation were left up to federal, state, and local health-care agencies. What resulted was a collection of state and local agencies and systems that grew in complexity as the program expanded. It went from administering six vaccines, at an annual cost of US$200 million in 1994, to administering 12 vaccines today at a cost of $3 billion. (See Exhibit 1.) The program made available the recommended immunizations from birth through 18 years of age. The program encompasses the ordering, distribution, and funding of vaccines for children all over the nation.
Over time, with expansion of the program, VFC grew into a sprawling supply chain. And, as with any complicated supply chain, product did not always flow smoothly to its destination. There were occasional problems with funding, supplies, storage, and delivery, any of which could stop the system cold. Managers of supply chains always worry about disruption. If there’s a hiccup in the system, it’s not just the flow of books, potato chips, or pajamas at stake, but the livelihood of everyone involved in the system. But that’s business. Consider the risk when a supply chain carrying vaccines is disrupted. Children’s lives are at stake.
Therefore, at the CDC, even a minor disruption of the VFC program was unacceptable. Thus, in 2003, the agency set out to revamp and strengthen the program. Now, five years later, it is rolling out the Vaccine Management Business Improvement Project (VMBIP), which promises to make programs like VFC more reliable, efficient, and transparent. The project has meant gargantuan change within the CDC and in public health agencies across the United States. From a business perspective, the new supply chain shares attributes of Wal-Mart Stores Inc., Amazon.com Inc., and the Social Security system. By employing best practices from a variety of successful organizations, VMBIP promises to save U.S. taxpayers hundreds of millions of dollars. “From a health-care perspective, the system’s highest purpose is very straightforward,” says Bill Gimson, the CDC’s chief operating officer. “Make it so that when doctors and nurses open up their refrigerators, the vaccine is there.”
Much depends on the success of VMBIP. If it can accomplish all its goals, VMBIP stands a good chance of becoming a blueprint for other federal health-care programs, and perhaps for other types of federal programs that distribute vast quantities of goods across the country, such as food programs and emergency relief. This business approach represents a shift within the CDC; its success will prove to skeptics that employing practices from the private sector can profoundly and effectively change the way government delivers goods and services.
Diagnosing the Problem
Just as sure as humidity would blanket Philadelphia every July, Kim Lane, a manager for the CDC’s national immunization programs, would get an e-mail informing her that once again, the City of Brotherly Love’s vaccine line of credit was running low. Philadelphia’s public health vaccine stock would soon be depleted. The message, coming as it always did on the cusp of the school year, would set off an emergency response within Lane’s team at the CDC.
Lane possesses an intensity and a 90-yard stare that make it clear when things need to be cranked up a few notches. Every July, things got cranked up. Philadelphia always got its money and vaccine — Lane and her team made sure of it. “But it drove me crazy,” Lane says, shaking her head inside her small office in the CDC’s Atlanta headquarters. “I just remember thinking, ‘There has to be a better way of doing this.’” But from Lane’s perspective, as the VFC program grew, tracking the physical distribution and ensuring proper storage of the lifesaving vaccines across the entire United States was becoming more and more problematic. Philadelphia wasn’t the only place with difficulties. There were literally hundreds of points of potential failure in other cities and states, any one of which could, and often did, interrupt the regular flow of vaccine to VFC grantee offices.
To take on the challenge of revamping a decade-old system involving every state and U.S. territory, tens of thousands of doctors, thousands more health-care workers, billions of dollars in funding, and the health of millions of children takes courage. To attempt it within the federal government takes persistence. To pull it off takes imagination, persuasiveness, and, above all, the rare ability to translate a felt need into actual results. Lane possessed all of those qualities. In 2003, she proposed to her boss, Bill Gimson, that the agency borrow from the best practices of both private industry and government to build an entirely new system — a new supply chain, really — for vaccinating children.
When dealing with a health-care program with the breadth and depth of VFC, few people have the capacity to see the scope of what needs to change or why it needs to change at all. Lane, Gimson, and the people they pulled into the VMBIP project early on had that dual perspective. Some had spent decades working for the CDC, both in the field and at the Atlanta headquarters. They knew that the problem wasn’t that children lacked access to vaccine; the vast majority did have access. The problem rested with the vaccine delivery system, which was outdated and overburdened and which relied on the superhuman efforts of thousands of health-care workers to function, especially in an emergency.
For Lane, the defining moment occurred on a cold night in January 1996, when she was assigned to the CDC’s office in Harrisburg, Pa. A basement alarm in a downtown building prompted a 3 a.m. phone call to her home. A recording on the line screeched, “Alert! Alert! Alert!” letting her know the refrigerator in the state health building had failed and the precious vaccine inside was about to lose its viability and be rendered useless. Lane, eight months pregnant with her first child, and her husband piled into their Chevy and negotiated the icy streets to the state health building. Lane’s husband worked until dawn moving boxes of vaccine from one refrigerator to another while she supervised. Everything was saved.
Lane knew she and others could not always count on an alarm to tell them the vaccine was at risk. While coordinating the children’s vaccine program in Puerto Rico in the 1980s, Gimson and his co-workers devised additional methods to keep close tabs on their vaccine-packed refrigerators and freezers. With an eyedropper, they’d carefully place a dot of red food coloring in a butter dish filled with water and freeze it. If they came back after the weekend, and the red dot had changed shape in the block of ice, they knew the electricity had been down for a time and the vaccine in the freezers might be compromised. The problem of unreliable power and faulty refrigerators persisted in these fragmented distribution centers.
Funding Woes
When the VFC program was first rolled out, each state health department and those in a handful of larger cities, 64 in all, acted as independent distributors of vaccine. They operated their own storage depots, where the vaccine was to be stored at the proper temperature. More than 430 depots were spread across the country. At the county and municipal level, orders for vaccine were received from 44,000 sites and more than 100,000 pediatricians and clinicians. Some orders were approved at the county or city level before they were forwarded to the state for another step in the approval process. The CDC approved bulk orders from the states and some large cities. It was also responsible for allocating money several times a year on the basis of the requests received from the 64 immunization projects. Funding consisted of 64 lines of credit against which vaccine could be purchased. Several times a year, the states and cities that were responsible for these lines of credit would make their pitch to the CDC for grants and ultimately the vaccine they thought they needed to serve their population.
Each of the 64 projects managed the VFC program differently. For example, some states required that a doctor’s representative pick up the vaccine at a local storage depot. Other program managers, like those in Chicago, had a crew deliver the vaccine to providers’ offices. Still others contracted with third-party commercial distributors to drop off the vaccine where it would be used. Orders were received from doctors’ offices by fax, phone, and U.S. mail. The turnaround time from order to delivery varied from hours to weeks, depending on the intended location. Some states, like Washington, offered universal vaccine coverage, which meant every child could receive vaccine from the VFC program. Others, like California, restricted access to the program; only children without private insurance were eligible.
For all that variability, thanks to the heroic efforts of thousands of public health workers, children were getting vaccinated in greater numbers than ever before. But as the years went on and new vaccines were added to the program, getting children immunized through VFC took more and more effort. As a result, the fragile system was easily thrown off the rails. By 2000, the time required to fill a state’s request for money had increased to six weeks. “That kind of wait time was normal,” Lane says. “It was all these legacy systems and approvals that we had put in place.” Says Gimson, “We had built an elephant.”
The program might have continued in the same form were it not for a confluence of events, including those of September 11, 2001. Those attacks, followed by anthrax scares, the greater threat of bioterrorism, and, later, pandemic flu fears drove the public to demand greater responsiveness of every government agency, including the CDC. CDC officials had to face the fact that the variability in the system made it impossible for them to get a clear and comprehensive picture of the overall vaccine landscape.
From 2000 through 2002, the nation went through an unprecedented period of vaccine shortages. First there were shortages of vaccine for flu, then for chicken pox, and, in 2002, a shortage of a new vaccine to prevent ear infections. Some doctors had plenty; some providers had none. “It was very clear there was this inequity, but we couldn’t tell how much vaccine was in the pipeline,” says Lane. “We had to rely on the states for answers, which meant we had to literally ask all 64 project offices to fax us their inventory logs so we knew approximately how much supply was out there.” Meanwhile, congressional representatives were on the phone, asking, “Why can’t children in my state get this vaccine?”
It became evident that if there were a nationwide emergency — a bird flu epidemic, for example — that put pressure on the vaccine supply, the CDC wouldn’t be able to respond the way the public would demand. “We had hundreds of depots, with six-plus months of vaccine inventory, in this very disjointed system, making it difficult to track,” Gimson says. “If we’d had a national crisis at that time, it would have been extremely challenging to locate and secure measles, rubella, or influenza vaccine and ship it to a centralized place in order to triage it appropriately.”
By July 2003, the CDC began addressing the key components of a new vaccine supply chain system. Lane was chief operating officer for what was then called the National Immunization Program, responsible for all the activities and systems that kept it running. It was clear to everyone that, at a minimum, the IT system created in 1993 to support VFC needed to be changed. A request for proposals was issued to replace the antiquated system and software. “But I was thinking that this could be our one opportunity to get it right, to think about where we want to be 10 years from now,” says Lane. “I had to ask, ‘What could we do differently knowing what we know today?’ From my perspective, we needed to launch a comprehensive program to look at all aspects of vaccine management at the CDC, not just this one part.”
That’s when Lane went to Gimson. “We started putting the jigsaw puzzle together, and we realized that the impact of a more comprehensive approach was going to be huge compared to the impact of changing one little ordering system or some other improvement to a single component,” Gimson says. He gave Lane the green light to look at revamping not just ordering, but the process in its entirety. And almost as soon as that happened, Lane was on the phone to Brock Lamont, a seasoned public health advisor in the CDC’s immunization program.
Improving the Process
Lane and Lamont had worked together in the late 1990s on the VFC program. If Lane was going to pull off any significant change, she knew she would need to sell the plan to a host of constituents, including CDC scientists and physicians and U.S. government officials all the way up to the Office of Management and Budget. Lamont, who was running the Houston VFC program, was a bridge to many of those parties, especially the state and local immunization programs.
After a three-month study, Lane and Lamont developed an initial set of recommendations for tackling the complexity and inefficiency of the current system. As much as possible, they borrowed from the best practices of supply chain management in private industry.
The chief components covered four main areas. The first was centralized distribution by a commercial third-party company. No state or city would store or deliver its own vaccine. The responsibility would belong to a third party, with performance guarantees and insurance against loss built into a contract. Having just a few centralized depots would significantly lower the amount of surplus vaccine in the system and save tens of millions of dollars in the purchasing cost.
To address the six-week funding bottleneck, the plan called for allocating money from a single large account directly to vaccine manufacturers, rather than through the 64 lines of credit. To bring a little Amazon.com-like efficiency to the process, the plan also called for a new Web-based ordering system that would be accessible to the 64 projects and to thousands of doctors’ offices. Not only would these changes bring the ordering procedure into the 21st century, but they would also provide a trove of data for scientists, health-care workers, and manufacturers, who could then analyze demand and other patterns of need and behavior. The system would also, for the first time, allow federal budget specialists to precisely reconcile the money spent with the actual amount of vaccine delivered. The final major change was the establishment of a centralized stockpile of vaccine in case of emergency.
Lamont didn’t need to be sold on one of the biggest shifts in the recommended system for distributing vaccine — centralized third-party distribution. He had already seen in Houston the benefits that could be gained from outsourcing that part of the process. When Lamont had first arrived in Texas, Houston had been doing all of the picking, packing, and distributing of vaccine to providers. Over time, however, the process had shifted; the VFC program began contracting all of that work to a commercial provider, a company that handled much of the third-party distribution throughout the country. Lamont rapidly became a fan. “The risk of losing millions of dollars of vaccine if your refrigerator goes down becomes someone else’s problem, and that is a huge benefit,” says Lamont. “We also didn’t have to maintain the delivery trucks and vans. All these benefits combined meant we could focus all our attention on health care, on improving immunization rates, and other things we didn’t always have the time to do before third-party distribution.”
 |
|
CDC veteran Kim Lane, pictured here
at the organization's offices in Atlanta, proposed the supply chain overhaul in 2003. Photograph © John Nowak |
“Of course, government can take only so many cues from business practices,” says Gimson. “We can look at what Wal-Mart does in pushing its suppliers hard to create their just-in-time system, but we can’t take it that far. We need more of a cushion than just-in-time. In other words, the government can’t become Wal-Mart, because often, as in this case, the stakes are too high.”
Lane, Gimson, and Lamont did have cost savings in mind when they were looking at changes to the children’s vaccination program. But they clearly understood that cost savings could not be the chief rationale for change. Saving and enhancing children’s lives through immunizations had to be the primary goal. They needed to show stakeholders that what they were proposing clearly accomplished that goal, and that along with it came these other benefits — cost savings, transparency, better data for scientists, and successful new vaccines. The stakeholders at the state or city level were not as interested in learning that the federal government was going to save more than $500 million by changing the program as they were in knowing that they would never run out of money for vaccine for the children in their state, and that they were guaranteed to get the needed funding within a certain number of days. Still, none of the stakeholders could deny that the financial performance of this initiative was head-turning: As of early 2008, its overall return on investment is estimated at $400 million, with annual savings in perpetuity of $19.5 million beginning in 2012.
Getting buy-in from every interested party was key to getting the program off the ground. Lane and Lamont wanted to avoid backlash at all costs; they needed a plan that included input from everyone. “One of our greatest concerns was that someone would try to torpedo the project by going to leadership and raising a lot of red flags,” Lamont says. “We tried to avoid that by engaging folks as early as we could. We had them at the table with us as we were developing these recommendations. And we showed the ability to listen to feedback.”
Lamont had the idea to schedule four regional town hall meetings to reach out to all 64 of the state and municipal vaccine distributors. In order for Lamont to present the recommendations and get feedback from the health-care specialists, the town hall meetings took place over the course of a day and a half. They were usually held at a small hotel near an airport to make the meetings as accessible as possible. At these meetings, he would briefly lay out the ideas to a group of about 20 to 25 people.
 |
|
Seasoned public health advisor Brock Lamont had become a fan of centralized
third-party distribution. Photograph © John Nowak |
Displaying diplomacy, Lamont was careful to be respectful of the miraculous results that state and local health workers had already accomplished in getting their immunization programs to function as well as they had. Feedback that Lamont heard over and over again from the program managers was that the team’s initial idea of deploying a centralized call center for the centralized distribution system was terrible; accepting vaccine orders from doctors’ offices was one of the few direct points of contact the managers had with health workers. Without this regular opportunity for contact with health workers, they worried that whatever relationship and leverage they had developed to promote new vaccination programs (ultimately to do their job of improving immunization coverage) would be diminished. Lamont heard the complaint and relayed the message. The call center concept was killed.
To cover all their bases, Lane and Lamont briefed the four vaccine manufacturers involved in the program, as well as the American Academy of Pediatrics and the Association of Immunization Managers. By the fall of 2004, Lane, Lamont, and the rest of the team were ready to start implementing their plan. Initially, that meant finding a vendor that could handle the centralized distribution system. Given its size, the procurement took more than a year. One of the key players in that process was Reginald Mebane, the chief management officer in the CDC’s Coordinating Center for Infectious Diseases.
Mebane came to the CDC after 25 years in operations with the FedEx Corporation. Lane would fall back on Mebane’s private-sector expertise again and again. While some health workers were worrying about such third-party details as where the company would position the thermometers in the refrigerators, Mebane hammered away on operations and the critical issue of good customer service. “My background is operations management, so my number one concern is answering questions like, What do you do to make sure you have continuity of operations?” Mebane says. “What is your plan for recruiting and making sure that your workforce is in place? What if the subcontractor you’re working with has a strike? How do you ensure good customer service? How do you take calls after hours? How do you deal with customers when you don’t have power? It really doesn’t matter whether it’s government or private sector, the concept is still the same.” In late 2006, with the input of Mebane and others, the multimillion-dollar, five-year contract for the distribution of an estimated 72 million doses of vaccine per year was awarded to McKesson Specialty, a business unit of McKesson Corporation based in Texas.
When McKesson decided to go after the VFC business, the team sent to pitch the company’s qualifications was well prepared to discuss the intricacies of supply chain management. After all, this was a division of a Fortune 50 company with customers that included the Department of Veterans Affairs and Wal-Mart. McKesson Specialty, a relatively young and small part of the corporation’s business, focuses on the just-in-time distribution of fragile and highly valuable drugs to private hospitals and physicians. So taking on the distribution of fragile children’s vaccine was, in theory, very similar to what the division was already doing for its private-sector customers. However, the team from McKesson quickly found out that working with the CDC was going to be unlike working with Wal-Mart or any other private-sector customer with which they had experience.
“We had to prove ourselves every step of the way,” says Dale Strimple, McKesson vice president. And they did, again and again. The due diligence process far exceeded anything that McKesson Specialty had experienced with its private-sector customers, says Phil Bolger, McKesson Specialty vice president and general manager. “They hit us with every scenario, every what-if they could come up with.” In the end, all that grilling paid off for McKesson, which won the contract and then some. It was a huge milestone. By November 2006, McKesson was officially in business, and Lane and her team could start thinking about rolling out their long-awaited plan.
Here too, the difference between government and the private sector came to bear. The big question was how quickly the CDC should transition the projects to the new McKesson centralized distribution contract. Mitchell Cohen, M.D., director of the CDC’s Coordinating Center for Infectious Diseases, wanted a much more measured approach to ensure the success of the program. First, there would be a two-month period of pilot programs in Maryland, California, Chicago, and Washington State to test the assumptions and fine-tune the program. Then the remaining 60 projects would be rolled out over a period of 13 months, with the last projects going live in June 2008. Cohen pressed the point that they could not afford to make mistakes. Sure, they could have rolled the program out in six months and saved money, “but the things that are higher priorities to us are being effectively able to get children immunized,” Cohen says. “That’s going to be the outcome that will reduce disease.”
Jan Hicks-Thomson, program manager for Washington, has felt some of the pressure ease up on her state’s children’s immunization program since it went live with the new system in February 2007. Like other vaccine distributors, Washington had its share of funding shortages, missed shipments, and refrigerator emergencies. Hicks-Thomson has had to deal with them all.
Washington delivers about 2.6 million doses of vaccine a year. During flu season under the old system, Hicks-Thomson and the rest of the state’s staff spent endless days and nights picking and packing influenza vaccine. “There were never enough people to do the job of managing vaccine inventory and furthering the goals of the state’s child immunization program,” she says. “I think under the new system we can focus more on promoting the best standards of health-care practice rather than running a warehouse.”
That doesn’t mean there haven’t been missed shipments of vaccine or other annoyances. Hicks-Thomson doesn’t like that shipments now come in many small boxes, rather than the large containers that providers used to get. But on the whole, the process is going well. Vaccine shipments arrive, on average, six to eight days from when the order is approved. “Generally, my local health departments are pleased,” she says. “We’re able to have more time for site visits, quality assurance — things we couldn’t do before.”
At the CDC, the progress of the VMBIP program is being monitored daily by dozens of people. One team is focused on setting up a provider and grantee “economic order quantity” system to minimize the costs associated with vaccine ordering and distribution, and others are using a weekly dashboard to track such key distribution performance indicators as the timeliness of vaccine shipments and the integrity of vaccine cold chain compliance.
One difficulty for the McKesson team was learning the vagaries of the tens of thousands of providers. For example, a doctor’s office might be closed for a few hours during lunch, or open only on certain days. The McKesson team would need to schedule deliveries to avoid the risk of having the vaccine go bad as it warmed up in the back of a truck. In Chicago, the McKesson team can’t use FedEx for delivery; it’s a nonunion company. If team members get these details wrong, they run the risk of inadvertently destroying vaccine. “Hospitals don’t shut down. Wal-Marts rarely do. We are not used to all these different schedules and procedures,” Strimple says. “As a result, we are revising many of our procedures, so we know what can be done and how to do it better.”
All the hard work is starting to pay off in exactly the ways the team first envisioned. For example, when the Tennessee Immunization Program needed an emergency shipment of hepatitis A vaccine on the Friday of Memorial Day weekend in 2008, vaccines were delivered the very next day. That the process ran so smoothly is a tribute to the work of the CDC and McKesson.
The lessons learned from the success of implementing VMBIP may prompt the CDC to change the way other public health procurements are managed, such as the ordering and delivery of laboratory supplies or distribution of vaccine and other medications in response to pandemic disease or other emergencies. Gimson, with great pride in the already evident success of the project, is looking forward to seeing how this venture will grow and perform in the future, and how it can improve the overall way government delivers service to the public. “I think it’s inevitable that something will happen down the road — there will be a shortage of vaccine, there will be a manufacturer’s disruption, there will be something,” Gimson says. “I am not looking forward to the day of crisis, but when it comes, we will be thanking our lucky stars this system is in place.”
VMBIP has already proved itself invaluable, but challenges remain. The team continues to work on issues of efficiencies, performance consistencies across the supply chain, communications, and accountability — no surprise given the mind-boggling complexity of a program like VMBIP, which reaches into so many different types of communities and involves so many interrelated processes. Even so, the program’s early operational success has had a profound effect on the organization, transforming its view of the potential benefits and feasibility of change.![]()
Reprint No. 08307
Author profile:
Michael V. Copeland is a senior writer at Fortune magazine in San Francisco. He received a 2006 Business Journalist of the Year award from the World Leadership Forum.