The Next Big Technology Could Be Nanomaterials
Discoveries surrounding a new class of impossibly small and improbably powerful compounds could reshape the materials industry — and the world around us.
When 3M introduced Scotch tape in 1930, at the start of the Great Depression, it was a remarkably apt product for a scrimping and saving population — and an extraordinary advance. By combining two recent discoveries, masking tape and DuPont’s impermeable cellophane, 3M scientists had produced a clear, clean, inexpensive binding device, useful for mending rips in a wide spectrum of materials. Musicians used Scotch tape to patch ripped sheet music; women, to fix broken fingernails; accountants, to restore torn ledger books; and banks, to repair ripped currency.
Today, most of us take this enormously durable and useful product for granted. It clutters up our kitchen junk drawer, an early example of the robust innovation culture that once powered the chemicals and materials industries. Between the mid-1920s and 1970s, corporate researchers created a steady stream of breakthrough materials. Cellophane was one, but the most consequential materials were synthetic polymers — complex molecules manufactured by humans. Many of them made possible new classes of commercial products that became enmeshed in our daily lives: nylon, latex, synthetic resin, Bakelite, the synthetic fibers used in contemporary clothing, polyvinyl chloride, polyethylene, polyurethane, and silicon.
So relentless was the wave of innovation that in an iconic scene in 1967’s The Graduate, actor Walter Brooke gave Dustin Hoffman’s drifting character a single word of career advice: “Plastics!” But right about the time the movie came out, the wave began to subside, owing to a range of factors. The growing level of concern about the environmental and health effects of synthetic chemicals, the leveling off of the market for new products in industrial countries, the expiration of patents, the rising costs of R&D, and the shrinking number of new hit polymers all curbed the enthusiasm for new investment. In addition, rising pressures for short-term profits pushed large corporations away from long-term basic research. And so, as the chemicals industry matured, it increasingly focused on commoditization (and competition based primarily on price), consolidation, and the search for new markets in emerging economies.
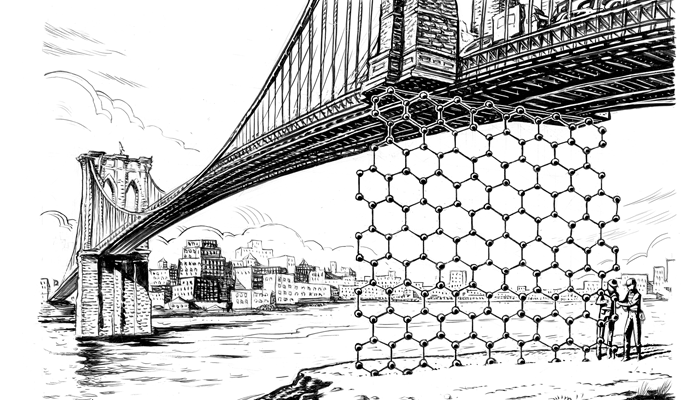
It was left to university labs to develop the breakthrough that kicked off the next renaissance in chemicals and materials manufacturing: the extraction of graphene, the first two-dimensional material. After more than 10 years of academic research and development, that singular breakthrough is producing scads of new materials that are impossibly thin and improbably strong, both in the graphene carbon family and from other 2D molecules. So far, the large incumbent chemicals companies have mostly ignored this hive of activity. But sitting back and watching may be a luxury they can no longer afford. It appears inevitable that these materials, which possess unique properties and versatility, could radically change the physical infrastructure, and the look and feel and quality, of the world around us. As was the case with the advent of the personal computer and the Internet, developments are moving so quickly that the companies that catch the wave early, whether they are prominent today or not, could greatly influence the future.
A Pencil-Thin Material
Oddly enough, cellophane tape helped make this breakthrough possible. On a Friday afternoon in a University of Manchester laboratory in 2004, Russian émigrés Andre Geim and Kostya Novoselov were playfully passing time while investigating the electrical properties of carbon graphite — the writing end of a so-called lead pencil. The two physicists had just about given up on making any progress when they decided to test whether they could obtain thinner flakes of graphite by peeling off a layer with Scotch tape. (They had seen other researchers use the tape to clean graphite before putting it under a microscope.)
On the first pass, Geim and Novoselov pulled off hundreds of layers of carbon without disturbing its underlying structure. Intrigued, they used more tape to repeat this process again and again. Before long, they had peeled the graphite back to its thinnest possible arrangement: a layer composed of a single atom. Here was a material so minute that it could be described as two-dimensional, a stunningly elusive concept that scientists had been chasing for years.
Graphene fits under the broad umbrella of nanomaterials, a category that encompasses products up to 100 nanometers thick. For comparison’s sake, the thinnest human hair is about 80,000 nanometers thick. But graphene has uncommon characteristics that enable it to perform wonders. With carbon atoms arrayed in a tightly packed honeycomb lattice, graphene is stronger than steel and more flexible than a rubber band. It conducts heat and electricity. It is transparent, virtually weightless, and impermeable to gases and even bacteria.
With carbon atoms arrayed in a tightly packed honeycomb lattice, graphene is stronger than steel and more flexible than a rubber band.
When Geim and Novoselov won the 2010 Nobel Prize in Physics for their breakthrough, the academy offered this simple description of graphene’s awesome power: A graphene hammock measuring one square meter tied between two trees could hold a four-kilogram (nine-pound) cat without breaking. “The hammock would weigh less than one milligram” (an ounce is 28,350 milligrams), the committee wrote, “the weight of one of the cat’s whiskers.”
Meet the Nanomaterials
Graphene is only one of many nanomaterials being examined at thousands of academic research labs around the world. In the molecule-sized world where they are developed and studied, these materials, compared with previously known materials, not only look different but act and react differently. And their potential applications go far beyond providing a wispy platform for catnaps. It is possible they can address some of humanity’s most vexing problems. The possibilities include breakthroughs in renewable energy and pollution reduction; in treatment of diseases and congenital conditions; in computer, sensor, and robotics technology; in manufacturing; and in new forms of clothing and packaging.
One of the most promising post-graphene nanomaterials is molybdenum disulfide (MoS2). In its ordinary form, MoS2 is a common lubricant, used in motorcycles and bicycles, and a component of nylon and Teflon. Thinned down to a single layer, MoS2 is a strikingly powerful light source, potentially usable in a solar cell array or in fiber-optic networks. It can convert even minute amounts of light into photovoltaic energy.
Similarly intriguing are silicene and phosphorene (derived from silicon and phosphorus, respectively). Both are malleable high conductors, shown to improve the performance of solid-state electrical circuits. These elements can combine chemically with larger, 3D molecules, and hence are useful in a variety of forms and objects, including computer and telecommunications components.
Hundreds of research papers are now published each year describing nanomaterial experiments in a range of fields. In manufacturing, for example, using a combination of heat and pressure, MIT researchers have created a 3D material from small flakes of graphene, opening the door to “fattening up” graphene so it can be used in construction and in durable goods. Items produced through this process would be 5 percent as dense as steel but 10 times as strong, according to MIT’s simulations — and could be tooled in a 3D printer. Unlike components printed from plastic and most metals, those printed from graphene particles conduct electricity, which makes them an attractive option for the bodies and components of next-generation electric automobiles and planes.
Some researchers think nanomaterials could be useful in mitigating the greenhouse effect and thus slowing or stalling climate change. Scientists at the University of Texas at Dallas have developed a honeycomb-shaped structure built from nanoscale molecules that can trap carbon emissions in the atmosphere. To keep gases from leaking out of the device, the researchers capped its outer surface with vapors of a one-nanometer molecule called ethylenediamine. This protective layer was an attempt to mimic the way bees seal parts of their hives with wax.
Another application, developed at Rice University, uses sheets of graphene to recycle waste carbon dioxide into ethylene and ethanol. In a lab test, graphene showed the potential to reduce carbon dioxide by up to 90 percent, converting 45 percent of greenhouse gases into clean fuel.
Potential healthcare uses include silicon nanowires that can be absorbed by human cells. Scientists at the University of Chicago propose placing these nanowires deep into the circulatory system, where they could record the barely perceptible electrical communications among structures inside the cells, revealing minute mutations and dysfunctions, including the beginning stages of tumor growth. The nanowires could also deliver molecular drugs directly to targeted cells.
Graphene Frontiers, based in Philadelphia, has created a device that takes advantage of graphene’s heat and electrical conductivity to measure trace bacteria in bodily fluid. When an antigen in the patient’s sample binds to an antibody attached to the graphene, electrostatic and temperature changes on the nanomaterial can immediately be detected, signaling the presence of infection or cancer.
Because virtually every advance in computer technology over the past five decades has stemmed from miniaturizing components and chips and from the concomitant performance improvements, nanomaterials offer the possibility of an unexpected palette of faster, smaller, and more portable systems that can manage and store vast amounts of information. If we could combine impossibly tiny nanoscale parts that conduct electricity while dissipating performance-degrading heat and friction, we could see a new model for mobile and desktop systems that boot almost instantly and save data in nanoseconds during a system shutdown. We could have ultra-high-definition displays and more natural and responsive digital assistants and voice intelligence.
Nanomaterials also seem likely to yield breakthroughs in consumer goods. In 2016, researchers at Dublin’s Trinity College published a report on their efforts to blend three 2D nanomaterials — graphene, tungsten diselenide, and boron nitride — to print sheets of electronic circuitry that could be inlaid in virtually any consumer item and provide alerts, warnings, data, and entertainment. For example, food packaging could display a digital countdown to warn of spoilage; wine labels could tell people when a pinot grigio hits the perfect temperature; and drug containers could alert the pharmacist when they are ready to be refilled.
A cottage industry is rapidly forming around the 2D materials world. Dozens of startups, funded by venture capital money and research grants, have emerged to support the first commercially successful applications of these products, some of which are already on the market. To date, however, mainstream chemicals companies are not participating in this activity, despite the likely impact of 2D nanomaterials on their future products — and the fact that these materials are largely adaptations of chemicals taken straight from the periodic table.
This muted response reflects how much the business model of the chemicals industry has changed, more than five decades after the heyday of plastics discoveries. “Just 20 or 30 years ago, they had huge industry labs that did amazing advanced research. Those days are past,” says Rigoberto Advincula, professor of macromolecular science and engineering at Case Western Reserve University. “Now, they mostly just try to improve on their existing product lines. And probably the only way they can grow or innovate is to acquire another company that already has the technology or know-how that they are lacking.”
Tiny Beginnings, Big Trajectory
The dazzling range of beneficial applications that nanomaterials could make possible and their unfathomable properties could not be more remote from the concept of nanotechnology as first hatched. The word nanotechnology was coined in the early 1980s by MIT-educated engineer Eric Drexler. He proposed the development of infinitely tiny molecular assemblers: robots the size of a virus that could synthesize molecules atom by atom to produce self-propagating devices. Drexler chose the prefix nano as shorthand for nanometer, one-billionth of a meter; for context, a sheet of paper is about 100,000 nanometers thick. Drexler’s nanobots were patterned after human ribosomes, which receive instructions from RNA and then assemble sequences of amino acids to construct protein molecules.
Drexler’s proposal was immediately controversial, because the technology had potentially devastating side effects. Swarms of mechanical biologics could be released, for example, multiplying out of control and battling with flora and fauna for the planet’s resources. “Robotic industries would compete vigorously among themselves for matter, energy, and space,” wrote computer scientist Bill Joy in a frequently cited article in Wired, published in 2000. “Unable to afford the necessities of life, biological humans would be squeezed out of existence.”
But nanotechnology did not quite follow the path that Drexler envisioned, or that Joy worried about. Instead of focusing on minute molecular assemblers, most researchers were inspired by Drexler’s nano vision to develop new types of materials. The time was right for this kind of R&D: A host of “atomic force” microscopes and electron-level imaging devices were designed in the early 1980s that for the first time allowed scientists to observe molecular or cellular activity in precise detail.
Aided by this equipment, researchers produced the first wave of nanomaterials. In this phase, nanoparticles made of various combinations of gold, copper, carbon, silver, iron, and platinum, among many other elements, were extracted. By the mid-2000s, many of these materials were being used commercially, typically in industrial applications. These included lightweight parts for cars and jets; targeted drug delivery and cancer treatments; self-cleaning surfaces; regrowth of skin, bone, and nerve cells; and wearable health monitors. Annual revenue from the U.S. sales of nanotechnology-enabled products grew more than sixfold between 2009 and 2016, exceeding US$500 billion in 2016, according to the National Nanotechnology Initiative.
These materials, although valuable and now relatively easy to produce, still represented only transitional steps toward the genuine promise of nanotechnology. They are three-dimensional, are composed of multiple atoms, and are, hence, fairly dense elements — many of them are more than 50 nanometers thick, whereas graphene is only 0.1 nanometer. Comparing the two classes of nanomaterials is analogous to placing early mobile phones (three pounds, 10 inches long, 30-minute battery life, no camera, no GPS, no music; cost: US$4,000) next to today’s multifunctional smart devices.
Indeed, the first actual glimpse of graphene in its native state came during what might have been the last great finding of the initial nanomaterial era. In 1991, NEC Corporation engineer Sumio Iijima was examining a form of carbon in a hollow sphere, known as a fullerene, when he discovered, as he put it, “a new material with a long, thin appearance.” Dubbed a carbon tube thanks to its cylindrical structure, this material’s unique shape and structural qualities were intriguing. The tube was, at the time, the strongest substance ever found and could maintain a length-to-diameter ratio of up to 132,000,000-to-1, also significantly greater than that of any other material. Suddenly, researchers around the world were studying carbon tubes. And before long, Iijima and others learned the secret to its distinctiveness: It was made up of individual slices of graphene, solitary carbon atoms that naturally spun together during extraction to form an unbroken cylinder.
Here was the missing link to the real nanomaterial revolution. Researchers had finally burrowed down to the molecular level — i.e., to where they could see a single atom closely enough to segregate and extract it. But they didn’t have the wherewithal to do either of those things yet. Since then, the carbon tube has had a remarkable run as the most successful nanomaterial. Thousands of tons of it are produced each year, and it is used in baseball bats and golfing equipment, in automobiles and desalination plants, and in medical implants and targeted pharmaceutical treatments. John DiLoreto, president of nanotechnology consultancy NanoReg, says: “The nanotube brought us to the threshold; at that point, graphene and all the single-molecule substances were just past the door.”
Although more than a dozen 2D particles have been isolated to date, graphene is still the only one that is routinely culled for commercial applications. It is often used in protective coatings such as paints to improve adherence and reduce corrosion (some studies have found that graphene paint might last as long as 100 years without fading). Recently, James Briggs, a large European coatings and lubricant provider, inked a deal with the U.K.’s Applied Graph-ene Materials to produce a line of graphene-based primers.
Other startups are working on mixing graphene with existing polymers to improve strength, heat resistance, and flexibility. Montreal-based Group NanoXplore has patented a formula for producing graphene as a powder to combine with plastics for “lightweighting” components used in heavy equipment or even for clothing with built-in exercise sensors. To circumvent the reluctance of polymer producers to adopt a new material that would first have to be approved by their customers — such as automakers or textile companies — NanoXplore recently acquired its own factory for manufacturing graphene in the form of compounded materials or pellets that OEMs could more easily integrate into finished products themselves.
Although the large chemicals businesses have yet to participate in this nascent industry, a few established companies from other industries have joined in, eyeing the substantial profit potential of nanoscale materials. IBM has invested more than $3 billion in nano semiconductor research and has already produced the world’s smallest and fastest graphene chip. Samsung has filed more than 400 patents related to graphene, involving manufacturing processes and touch screens, among other things, and Samsung’s Advanced Institute of Technology has funded an effort to produce a blueprint for extending lithium-ion battery life using silicon and graphene. Apple is looking into adding graphene signal paths to its lightning connector. And one of the largest consumer goods companies has quietly earmarked funds for packaging and product design based on nanoscale molecules.
Great Expectations
To understand the conundrum faced by chemicals companies, it’s instructive to understand the evolution and elongation of their supply chains. For much of their history but especially since the 1960s, chemicals providers were known for innovation and new products. Their customers were generally other large top-of-the-pyramid companies: OEMs in the auto and aerospace, computer hardware, and textiles industries. By offering a steady stream of new materials, particularly plastics, that could be formulated to fit into assembled products and manufacturing processes, chemicals firms expanded their market presence.
But more recently, the supply chain has expanded to include a set of new suppliers such as polymer and additive makers that have usurped the traditional role of chemicals companies as innovators. These suppliers purchase feedstock from the chemicals companies and provide parts and materials to larger manufacturers, working with them to improve weight, technology, and performance. Which leaves chemicals companies in a bind. If they invest in new materials development in the 2D arena, which would require a long-term and expensive commitment with plenty of uncertainty, they run the risk of cannibalizing the business of some of their most important customers — namely, the suppliers that are themselves adopting graphene and other nanomaterials as a base for their products.
Of course, that could change. “If one of these nanomaterials proves to be an excellent fit for their traditional chemistry, let’s say making epoxy or making polyurethane, then I can imagine that the big chemicals companies will get more interested,” Advincula at Case Western notes. “Otherwise, they’ll leave it to the rest of us for now.”
In the meantime, the 2D sector will undoubtedly take a cue from Silicon Valley: moving forward with frequent flashy new innovations in the next three to five years, while accumulating knowledge about how these miracle materials operate. In describing the evolution of nanotechnology, Paul Higgins, Nano-Xplore’s chief operating officer, harks back to the way engineers tested new bridges in 1900. “They sent sheep across to see if the structure held up for two weeks before letting people go on it,” Higgins says. “They didn’t really know why the bridge stood up. That was where materials science was not long ago. But now we are working with these materials by design, experimenting with new ideas with some confidence about how they will respond and how we will be able to apply them to real-world issues.”
Indeed, if the outlook of researchers and startups is any indication, it won’t be long now before nanomaterials are as commonplace as Scotch tape.
Reprint No. 17306
Author profile:
- Jeffrey Rothfeder is a contributing editor of strategy+business. His most recent book is Driving Honda: Inside the World’s Most Innovative Car Company (Penguin, 2014).